Science
Antibody Therapy Shows Promise in Eradicating Multiple Myeloma
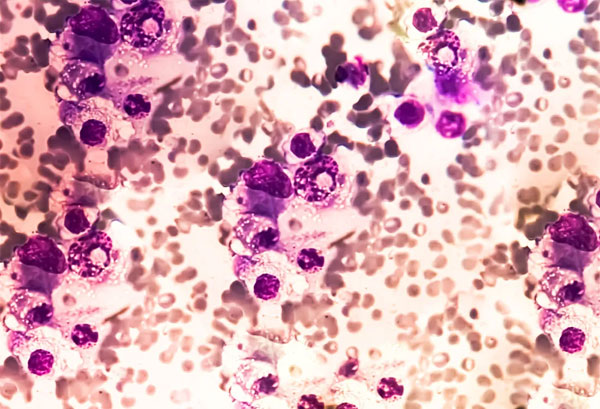
A novel antibody-based therapy has demonstrated the potential to eliminate residual traces of multiple myeloma, a serious form of blood cancer, according to interim results from a clinical trial. The study, which involved 18 patients, focused on the bispecific antibody linvoseltamab, which targets both immune and cancer cells. Notably, all participants showed no detectable signs of the disease following treatment, as reported by researchers at the American Society of Hematology (ASH) annual meeting held in Orlando, USA, on December 8, 2023.
Dr. Dickran Kazandjian, the lead investigator from the University of Miami’s Miller School of Medicine, highlighted the significance of these findings. “These patients received modern and effective, up-front treatment that eliminated 90 percent of their tumor,” he stated. Traditionally, patients in similar situations would undergo high-dose chemotherapy followed by a bone marrow transplant, procedures that carry considerable risks. Instead, the trial participants received treatment with linvoseltamab, which may offer a safer alternative.
Encouraging Results and Future Implications
The results from the trial are seen as “extremely impressive” by researchers, who noted that the disappearance of lingering myeloma cells suggests a robust immune response. This development raises hopes that linvoseltamab could enable eligible patients to avoid the complications associated with bone marrow transplants, potentially leading to long-lasting disease control. While the early findings are promising, researchers caution that the possibility of relapse remains.
Multiple myeloma originates from plasma cells, which are immune cells responsible for producing antibodies. When these cells become cancerous, they proliferate in the bone marrow, disrupting normal blood cell production and causing damage to various organs. Although advancements in treatment have improved patient outcomes, a definitive cure for this cancer has yet to be established.
Linvoseltamab operates by binding to two specific proteins: CD3, a marker on tumor-killing T cells, and BCMA, found on myeloma cells. This dual targeting facilitates a direct immune attack on the cancer cells, enhancing the body’s ability to fight the disease.
Some participants in the trial did report side effects, including neutropenia, characterized by low white blood cell levels, and upper respiratory infections. Nevertheless, Dr. Kazandjian noted that all adverse events remained within an acceptable safety range. Given its encouraging performance in the trial, linvoseltamab may eventually represent a significant advancement in the management of multiple myeloma, potentially leading to what researchers describe as a “functional cure.”
The ongoing research into this therapy highlights the need for continued innovation in cancer treatment, particularly for diseases like multiple myeloma, where traditional therapies may not provide adequate solutions.
-

 World5 months ago
World5 months agoSBI Announces QIP Floor Price at ₹811.05 Per Share
-

 Lifestyle5 months ago
Lifestyle5 months agoCept Unveils ₹3.1 Crore Urban Mobility Plan for Sustainable Growth
-

 Science4 months ago
Science4 months agoNew Blood Group Discovered in South Indian Woman at Rotary Centre
-

 World5 months ago
World5 months agoTorrential Rains Cause Flash Flooding in New York and New Jersey
-

 Top Stories5 months ago
Top Stories5 months agoKonkani Cultural Organisation to Host Pearl Jubilee in Abu Dhabi
-

 Sports4 months ago
Sports4 months agoBroad Advocates for Bowling Change Ahead of Final Test Against India
-

 Science5 months ago
Science5 months agoNothing Headphone 1 Review: A Bold Contender in Audio Design
-

 Top Stories5 months ago
Top Stories5 months agoAir India Crash Investigation Highlights Boeing Fuel Switch Concerns
-

 Business5 months ago
Business5 months agoIndian Stock Market Rebounds: Sensex and Nifty Rise After Four-Day Decline
-

 Sports4 months ago
Sports4 months agoCristian Totti Retires at 19: Pressure of Fame Takes Toll
-

 Politics5 months ago
Politics5 months agoAbandoned Doberman Finds New Home After Journey to Prague
-

 Top Stories5 months ago
Top Stories5 months agoPatna Bank Manager Abhishek Varun Found Dead in Well