Science
Maharashtra FDA Assures Safety Amid Coldrif Syrup Controversy
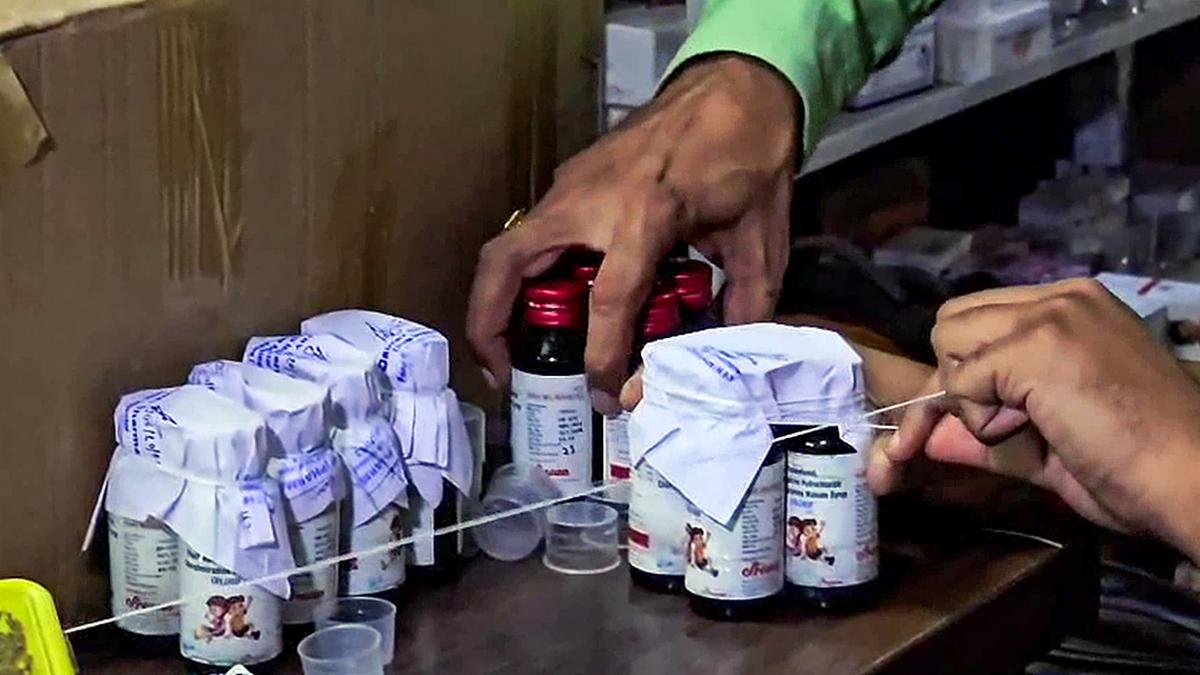
The Maharashtra Food and Drug Administration (FDA) reassured residents on October 6, 2025, that no Coldrif syrup has been distributed in the state. This statement follows alarming reports of child deaths in Madhya Pradesh and Rajasthan, which are allegedly connected to a contaminated batch of the syrup. The FDA’s swift response includes an urgent advisory issued on October 5, 2025, calling for an immediate cessation of sales, distribution, and use of Coldrif Syrup.
The specific batch in question, Coldrif Syrup (containing Phenylephrine Hydrochloride and Chlorpheniramine Maleate), is identified as Batch No. SR-13. Manufactured by Sresan Pharma in May 2025, this batch is set to expire in April 2027. Laboratory tests uncovered the presence of Diethylene Glycol (DEG), a toxic substance often found in industrial products, including antifreeze. Ingesting DEG can lead to severe health complications, including kidney failure, and has been associated with multiple fatalities.
Public Safety Measures and Manufacturer Accountability
In response to the crisis, Dr. D.R. Gahane, the FDA’s Joint Commissioner (Drugs), emphasized the safety of Maharashtra’s citizens. He stated, “We held a meeting with the Drug Control Authorities in Tamil Nadu to trace the distribution of the batch in Maharashtra. They confirmed that Coldrif cough syrups were not supplied in the State. It is difficult to trace whether someone travelled from Maharashtra to neighbouring States, bought the cough syrup and brought it here. Nevertheless, we issued the statement in the public interest and safety.”
The FDA has mandated that all pharmacies, hospitals, wholesalers, and license holders cease handling the affected batch immediately. They are required to report any remaining stock to local drug control authorities. Citizens are encouraged to report any possession of the syrup through a toll-free number, email, or mobile contact provided by the FDA.
To further mitigate risk, drug inspectors and assistant commissioners have been deployed to notify market stakeholders and ensure any remaining stock is frozen. The Central Drugs Standard Control Organisation (CDSCO) plans to take stringent action against the manufacturer and has requested Tamil Nadu’s FDA to assist in enforcing these measures.
Wider Implications and Government Response
The situation has prompted significant reactions across various states. Following the reported deaths of children in Chhindwara district, Madhya Pradesh has imposed a complete ban on the syrup. Rajasthan has suspended its state drug controller and halted the distribution of all medicines from another manufacturer, Kaysons Pharma, while establishing an expert committee to investigate the matter further.
In addition, the Union Health Ministry has advised that cough and cold medications should not be prescribed for children under two years of age, reinforcing guidelines to health departments across all states and union territories.
As the investigation unfolds, both the FDA and health authorities remain vigilant, emphasizing the priority of public safety in light of this troubling incident.
-

 World5 months ago
World5 months agoSBI Announces QIP Floor Price at ₹811.05 Per Share
-

 Lifestyle5 months ago
Lifestyle5 months agoCept Unveils ₹3.1 Crore Urban Mobility Plan for Sustainable Growth
-

 Science4 months ago
Science4 months agoNew Blood Group Discovered in South Indian Woman at Rotary Centre
-

 World5 months ago
World5 months agoTorrential Rains Cause Flash Flooding in New York and New Jersey
-

 Top Stories5 months ago
Top Stories5 months agoKonkani Cultural Organisation to Host Pearl Jubilee in Abu Dhabi
-

 Sports4 months ago
Sports4 months agoBroad Advocates for Bowling Change Ahead of Final Test Against India
-

 Science5 months ago
Science5 months agoNothing Headphone 1 Review: A Bold Contender in Audio Design
-

 Top Stories5 months ago
Top Stories5 months agoAir India Crash Investigation Highlights Boeing Fuel Switch Concerns
-

 Business5 months ago
Business5 months agoIndian Stock Market Rebounds: Sensex and Nifty Rise After Four-Day Decline
-

 Sports4 months ago
Sports4 months agoCristian Totti Retires at 19: Pressure of Fame Takes Toll
-

 Politics5 months ago
Politics5 months agoAbandoned Doberman Finds New Home After Journey to Prague
-

 Top Stories5 months ago
Top Stories5 months agoPatna Bank Manager Abhishek Varun Found Dead in Well









