Health
India Enforces Manufacturing Upgrades After Cough Syrup Tragedy
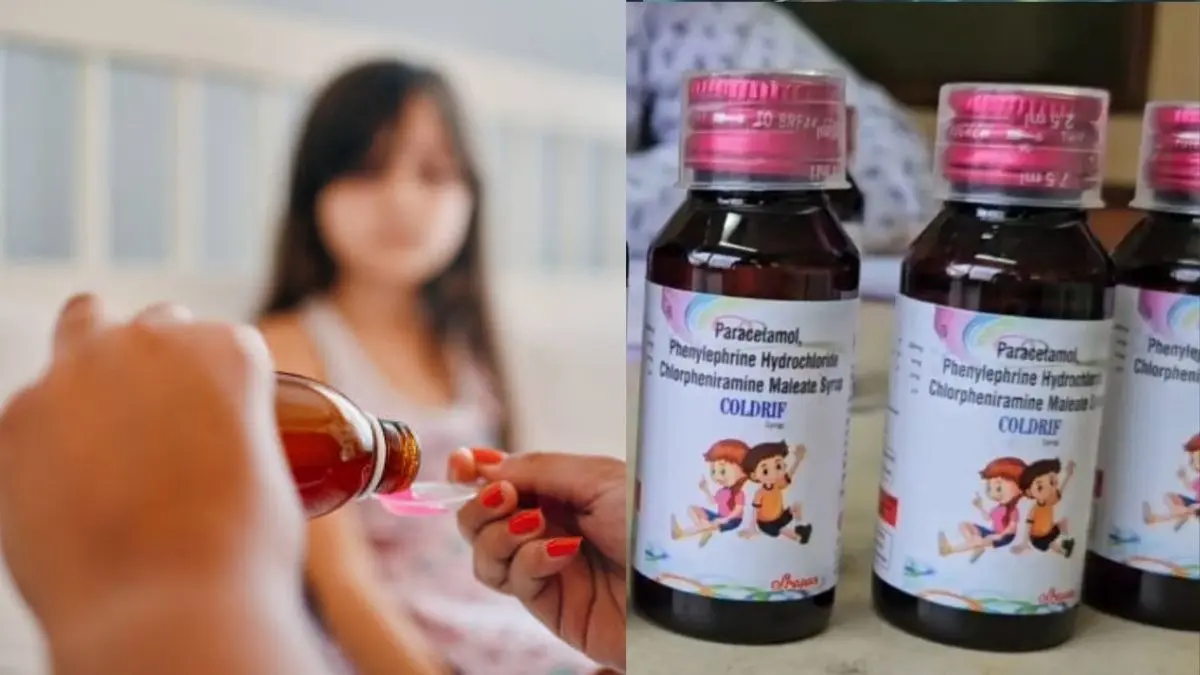
India has mandated drug manufacturers to upgrade their production facilities to international standards by the end of 2024, following a public health crisis linked to locally produced cough syrup. This decision comes in the wake of at least 24 child fatalities, including the tragic death of a three-and-a-half-year-old boy, which has sparked outrage across the nation. The Indian government is adamant about enforcing compliance with World Health Organization (WHO) recommendations to restore confidence in its pharmaceutical industry.
In late 2023, Indian authorities issued a directive requiring pharmaceutical companies to ensure their plants adhere to rigorous safety standards. This includes implementing protocols to prevent cross-contamination and enabling batch testing of products. The mandate was prompted by earlier incidents where Indian-made cough syrups were connected to over 140 child deaths in Africa and Central Asia, damaging India’s reputation as the “pharmacy of the world.”
While larger pharmaceutical companies have successfully met the June 2024 deadline, smaller firms were initially granted a 12-month extension, until December 2024. However, recent developments indicate that the government will not extend this deadline further, despite appeals from industry representatives citing potential bankruptcies due to the costs of compliance. The decision appears to have been influenced by findings that Sresan Pharmaceutical Manufacturer, the producer of the Coldrif syrup linked to the recent deaths, had not upgraded its facilities.
Testing revealed dangerously high levels of diethylene glycol (DEG) in Coldrif syrup manufactured by Sresan, with concentrations nearly 500 times the safe limit established by both India and WHO standards. DEG, a toxic substance, is sometimes used as a cheap substitute for more expensive pharmaceutical-grade solvents. In response to these findings, officials have moved to revoke Sresan’s manufacturing license and have initiated criminal proceedings against its founder, S. Ranganathan, for suspected manslaughter.
Udaya Bhaskar, a representative of the All India Drugs Control Officers’ Confederation, expressed concern that adherence to safety standards could have prevented the recent deaths. The affected syrup batch was produced in May 2023, and Bhaskar emphasized that the responsibility for ensuring product safety lies with manufacturers, not the government.
The crisis has triggered a renewed debate about the enforcement of safety regulations in India’s burgeoning pharmaceutical sector, which is valued at approximately $50 billion. The industry comprises around 3,000 companies and over 10,000 factories, with a significant portion of production attributed to small and medium-sized enterprises. Many of these businesses are apprehensive about meeting the new standards, fearing it could jeopardize their financial viability.
Jagdeep Singh, secretary of the SME Pharma Industries Confederation, warned that without an extension, nearly half of the manufacturing units in the pharmaceutical hub of Himachal Pradesh could close, leading to potential shortages and job losses. He noted that several companies have already begun discontinuing products due to the burden of compliance costs.
Despite these concerns, regulators have stated that the deadline cannot be postponed again, citing the urgency of ensuring public safety. A source close to the situation remarked, “People are dying,” highlighting the dire consequences of inaction.
As the community in Parasia, Madhya Pradesh, continues to grapple with the fallout from the drug safety lapses, local drug inspectors are conducting random tests of cough syrups in pharmacies. Several establishments selling Coldrif have been temporarily shut down for failing to provide adequate documentation regarding the syrup’s sale. Community health workers are also engaging in door-to-door initiatives to encourage residents to return any Coldrif products still in their possession.
The tragic story of three-and-a-half-year-old Mayank Suryavanshi underscores the personal toll of this crisis. After being prescribed Coldrif for a fever, Mayank’s condition rapidly deteriorated, ultimately leading to his death from acute kidney failure on October 9, 2023. His father, Nilesh Suryavanshi, lamented the loss, stating, “We never imagined a simple medicine could turn life-threatening,” and called on the government to ensure no other parents face such a tragedy.
In light of this scandal, the Indian Pharmacopoeia Commission (IPC) has started requiring manufacturers to test oral liquid medications for the presence of DEG and similar harmful substances before they can be sold. This initiative aims to prevent future incidents of contamination, which can occur through either deliberate adulteration or accidental mix-ups.
The current situation has drawn attention to the regulatory shortcomings that allowed substandard products to enter the market. Despite the serious nature of the violations, there have been no recorded legal consequences for manufacturers involved in the earlier fatalities abroad.
As the investigation continues, the Indian government faces mounting pressure to ensure that the pharmaceutical sector prioritizes safety and compliance. The tragic outcomes of this crisis may serve as a catalyst for change, prompting stricter oversight and more rigorous enforcement of safety regulations moving forward.
-

 World5 months ago
World5 months agoSBI Announces QIP Floor Price at ₹811.05 Per Share
-

 Lifestyle5 months ago
Lifestyle5 months agoCept Unveils ₹3.1 Crore Urban Mobility Plan for Sustainable Growth
-

 Science4 months ago
Science4 months agoNew Blood Group Discovered in South Indian Woman at Rotary Centre
-

 World5 months ago
World5 months agoTorrential Rains Cause Flash Flooding in New York and New Jersey
-

 Top Stories5 months ago
Top Stories5 months agoKonkani Cultural Organisation to Host Pearl Jubilee in Abu Dhabi
-

 Sports4 months ago
Sports4 months agoBroad Advocates for Bowling Change Ahead of Final Test Against India
-

 Science5 months ago
Science5 months agoNothing Headphone 1 Review: A Bold Contender in Audio Design
-

 Top Stories5 months ago
Top Stories5 months agoAir India Crash Investigation Highlights Boeing Fuel Switch Concerns
-

 Business5 months ago
Business5 months agoIndian Stock Market Rebounds: Sensex and Nifty Rise After Four-Day Decline
-

 Sports4 months ago
Sports4 months agoCristian Totti Retires at 19: Pressure of Fame Takes Toll
-

 Politics5 months ago
Politics5 months agoAbandoned Doberman Finds New Home After Journey to Prague
-

 Top Stories5 months ago
Top Stories5 months agoPatna Bank Manager Abhishek Varun Found Dead in Well









