Health
Gene Therapy Restores Immune Function in Children with Rare Disorder
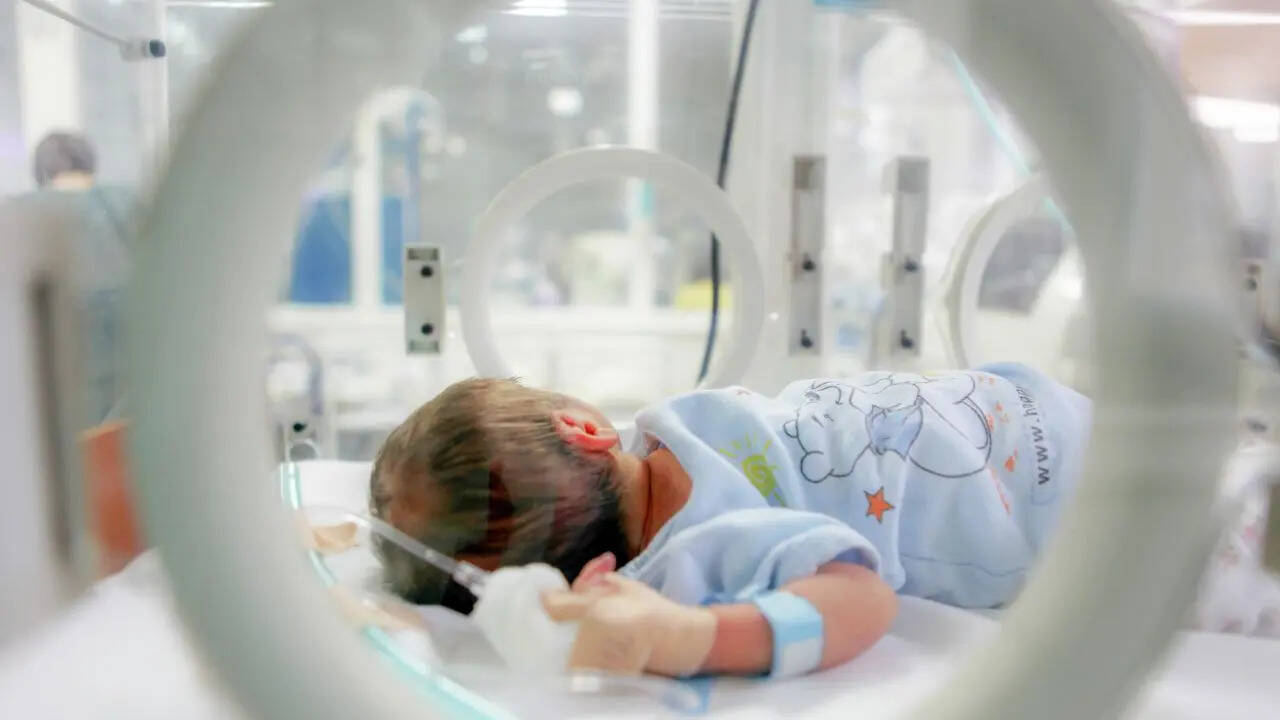
A recent study has demonstrated that an experimental gene therapy effectively restores immune system function in children diagnosed with adenosine deaminase deficiency severe combined immunodeficiency (ADA-SCID). Conducted by researchers from UCLA, University College London, and Great Ormond Street Hospital, this innovative therapy has successfully treated 59 out of 62 children affected by this rare and life-threatening genetic disorder, providing new hope in a field where traditional treatments often fail.
Understanding ADA-SCID is crucial to grasp the significance of this breakthrough. The condition arises from mutations in the ADA gene, which is essential for proper immune system function. Children suffering from ADA-SCID are extremely vulnerable to infections, facing serious health risks that can compromise their daily lives. Without effective treatment, many of these children do not survive past the age of two. Conventional therapies, including bone marrow transplants and enzyme replacement injections, have limitations and potential long-term risks, underscoring the need for more effective alternatives.
Mechanism of Gene Therapy
The gene therapy in question utilizes a modified lentivirus to introduce a healthy copy of the ADA gene into the blood stem cells of affected children. These stem cells are responsible for generating various blood and immune cells. Once the corrected stem cells are reinfused, they begin to produce functional immune cells capable of combating infections. Immune recovery typically begins shortly after treatment, although it can take between six to twelve months for the immune system to fully stabilize.
The findings of this study, published in the New England Journal of Medicine, represent the largest and longest follow-up of this gene therapy to date, accumulating a total of 474 patient-years of follow-up data. The results indicate that immune function among successfully treated children remains stable beyond the initial recovery phase, with no significant complications reported.
Positive Long-term Outcomes
Dr. Donald Kohn, a distinguished professor at UCLA and senior author of the study, expressed optimism regarding the results. He stated, “The durability of immune function and the continued safety profile are all incredibly encouraging.” Most adverse events reported were mild and associated with routine preparatory procedures rather than the gene therapy itself. Remarkably, only three patients did not respond positively to the treatment, but all were able to revert to standard therapies.
An innovative aspect of this therapy is the use of frozen preparations of corrected stem cells, which have proven equally effective as fresh cells. This cryopreservation technique enhances accessibility, allowing for local collection and processing at manufacturing facilities, and broadening the reach of this life-saving treatment.
The research team, supported by the California Institute for Regenerative Medicine, is actively pursuing FDA approval, aiming to make the therapy widely available within two to three years. The robust data gathered strongly supports this application, as efforts are underway to ensure that the treatment meets commercial pharmaceutical standards.
One particularly inspiring case is that of eleven-year-old Eliana Nachem, who received the therapy as an infant after spending her early years in strict medical isolation due to her condition. Today, she enjoys a normal childhood, attending school and participating in sports. Her parents described the infusion of the corrected cells as a “rebirth,” highlighting the profound impact that gene therapy has had on her life and on the lives of many children suffering from ADA-SCID.
This innovative gene therapy not only signifies a major advancement in the treatment of ADA-SCID but also holds the potential to pave the way for future developments in gene therapies for other genetic disorders, potentially transforming the landscape of pediatric healthcare.
-

 World5 months ago
World5 months agoSBI Announces QIP Floor Price at ₹811.05 Per Share
-

 Lifestyle5 months ago
Lifestyle5 months agoCept Unveils ₹3.1 Crore Urban Mobility Plan for Sustainable Growth
-

 Science4 months ago
Science4 months agoNew Blood Group Discovered in South Indian Woman at Rotary Centre
-

 World5 months ago
World5 months agoTorrential Rains Cause Flash Flooding in New York and New Jersey
-

 Top Stories5 months ago
Top Stories5 months agoKonkani Cultural Organisation to Host Pearl Jubilee in Abu Dhabi
-

 Sports4 months ago
Sports4 months agoBroad Advocates for Bowling Change Ahead of Final Test Against India
-

 Science5 months ago
Science5 months agoNothing Headphone 1 Review: A Bold Contender in Audio Design
-

 Top Stories5 months ago
Top Stories5 months agoAir India Crash Investigation Highlights Boeing Fuel Switch Concerns
-

 Business5 months ago
Business5 months agoIndian Stock Market Rebounds: Sensex and Nifty Rise After Four-Day Decline
-

 Sports4 months ago
Sports4 months agoCristian Totti Retires at 19: Pressure of Fame Takes Toll
-

 Politics5 months ago
Politics5 months agoAbandoned Doberman Finds New Home After Journey to Prague
-

 Top Stories5 months ago
Top Stories5 months agoPatna Bank Manager Abhishek Varun Found Dead in Well









